100% Petroleum
Natural Origin Index (ISO 16128): 0
Carbomer
Category of Ingredient: Rheology Modifier, Thickening Agent, Emulsion Stabilizer
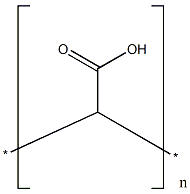
Chemical Structure:
Description of Synthesis and Manufacture:
Carbomer is a generic name for a family of high molecular weight, crosslinked polymers of acrylic acid. From a renewability standpoint, the defining characteristic of Carbomer is its primary feedstock: the monomer acrylic acid. The overwhelming majority of commercially available acrylic acid is produced via the two-stage vapor-phase oxidation of propylene. Propylene is a fundamental petrochemical building block derived directly from the steam cracking of hydrocarbons found in fossil fuels (petroleum and natural gas). While research exists regarding bio-based routes to acrylic acid (such as fermenting sugars to lactic acid and dehydrating it), these methods are not yet widely adopted for industrial-scale production of cosmetic raw materials. Therefore, the very backbone of the Carbomer molecule is sourced from non-renewable petroleum reserves.
The manufacturing process involves free-radical precipitation polymerization. The acrylic acid monomers are polymerized in a solvent medium along with a small percentage of a polyalkenyl polyether crosslinking agent (such as allyl sucrose or allyl pentaerythritol). Historically, benzene was the primary solvent used due to its efficiency, but due to severe toxicity concerns and regulatory pressure (ICH Q3C guidelines on residual solvents), modern manufacturing has largely shifted to less toxic solvents like ethyl acetate, cyclohexane, or co-solvent mixtures.
In layman’s terms: Think of a Carbomer molecule as a gigantic net made of thousands of smaller, identical links hooked together. The small links are called acrylic acid. Currently, the only efficient way to get the massive amounts of acrylic acid needed for global manufacturing is by processing crude oil. To turn these long chains into a useful gel rather than just slimy strings, “crosslinkers” are added during manufacturing. These act like small bridges connecting different parts of the chains together, creating a three-dimensional sponge-like structure.
The final step that makes Carbomer useful happens during cosmetic formulation, not initial manufacture. The resulting polymer produced above is acidic and tightly coiled. When dispersed in water and neutralized with a common base (like sodium hydroxide or triethanolamine), the carboxylic acid groups along the polymer backbone become negatively charged. These negative charges repel each other violently, forcing the tightly coiled molecule to unroll and swell immensely—absorbing up to 1,000 times its weight in water—which is what creates the viscous gel structure in creams and lotions.
References:
Todaro, A. et al. (2018). Carbomers: From Synthesis to Biomedical Applications. Progress in Polymer Science, 86, 1-23.
Lochhead, R. Y. (2017). The Role of Polymers in Cosmetics: Recent Trends and Future Views. Journal of Cosmetic Science, 68(3), 217–220.
Swift, J. A., & Paul, S. (2019). Polyacrylic Acid-Based Rheology Modifiers. In Cosmetic Science and Technology: Theoretical Principles and Applications (pp. 481-496). Elsevier.
Bauer, Jr. et al. “Acrylic Acid and Derivatives.” Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000.
Leave a Reply