100% Renewable
Natural Origin Index (ISO 16128): 1
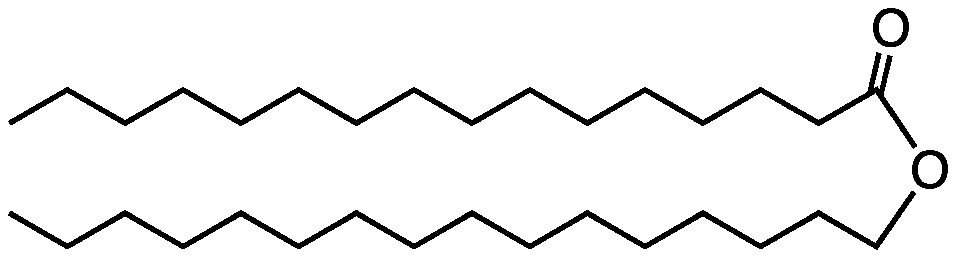
Cetyl Palmitate
Category of Ingredient: Emollient, Emulsion Stabilizer, Viscosity Controlling Agent, Occlusive
Chemical Structure:
Description of Synthesis and Manufacture:
Cetyl Palmitate is a waxy ester synthesized via the direct condensation reaction (esterification) of cetyl alcohol (1-hexadecanol) and palmitic acid (hexadecanoic acid). In modern cosmetic manufacturing, the feedstock for both precursors is overwhelmingly sourced via oleochemical pathways derived from renewable plant oils. Palmitic acid is a primary saturated fatty acid obtained through the hydrolysis (splitting) of triglyceride oils, predominantly palm oil. The corresponding cetyl alcohol is subsequently produced via the high-pressure hydrogenation of palmitic acid or its methyl ester derivatives using metal catalysts.
The industrial synthesis of the final ester involves charging these two long-chain aliphatic reactants into a reactor, typically in near-equimolar quantities. The reaction is usually catalyzed by strong acids (such as p-toluenesulfonic acid) or, more commonly in modern high-purity processes, metal catalysts like organotins or titanates at elevated temperatures (often 150°C–220°C). Because esterification is an equilibrium reaction that generates water as a byproduct, the process water is continuously removed—usually under reduced pressure (vacuum) or via azeotropic distillation—to drive the equilibrium toward the ester product according to Le Chatelier’s principle.
Once the reaction reaches the desired endpoint (indicated by a low acid value showing consumption of the fatty acid), the crude ester undergoes purification. This involves neutralizing or filtering out the catalyst, followed by bleaching with activated earth and steam deodorization to remove trace unreacted raw materials and color bodies. The final molten product is cooled and flaked or prilled into a white, crystalline wax solid.
In layman’s terms: Historically, this waxy ingredient was known as “spermaceti” and was harvested from sperm whales. Today, it is synthesized entirely using plant sources. Imagine taking two different types of fats derived from palm or coconut oil: one is a fatty acid (palmitic acid) and the other is a fatty alcohol (cetyl alcohol). In a chemical plant, these two distinct “building blocks” are heated together in a big tank along with a special “helper” chemical called a catalyst. This process hooks the two pieces together permanently to create one larger, waxy molecule, squeezing out water in the process. The resulting wax is excellent at giving creams thick body and locking moisture into the skin.+1
References:
- O’Lenick, A. J. (2014). Esters. In Surfactants in Personal Care Products and Decorative Cosmetics (3rd ed.). CRC Press.
- Gunstone, F. D. (2004). The Lipid Handbook with CD-ROM. CRC Press.
- Cornils, B., et al. “Fatty Acids” and “Fatty Alcohols.” Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000.
- ISO 16128-2:2017. Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients and products — Part 2: Criteria for ingredients and products. International Organization for Standardization.
Leave a Reply